Fabulous Info About What Is R In KJ

Decoding 'R' in Kilojoules
1. The Universal Gas Constant Explained
Ever stared at a physics equation and thought, "What on Earth does 'R' stand for?" If you're dealing with energy in kilojoules (kJ), and 'R' pops up, chances are we're talking about the ideal gas constant. And no, it doesn't have anything to do with pirates or buried treasure — though wouldn't that be a fun plot twist?
The ideal gas constant, symbolized as 'R', is a fundamental physical constant that relates the energy scale to the temperature scale for a mole of gas at a given pressure and volume. Basically, it's a bridge between the microscopic world of atoms and molecules and the macroscopic properties we can measure, like pressure and temperature.
So, while "R" might seem like a simple letter, its actually a powerhouse of information, quietly influencing how gases behave. Think of it as the secret ingredient that makes gas laws like PV=nRT actually... work! Without it, understanding things like how balloons inflate or why car engines can generate power would be much, much harder. It's the ultimate behind-the-scenes player in the world of thermodynamics.
And where does the kilojoule (kJ) come in? Well, many times 'R' is used when we are calculating energy changes related to gases, and those energy changes are often expressed in kilojoules. Understanding R allows us to determine how much energy is needed to heat a gas, compress it, or cause it to react. Thus, the kJ links "R" to practical application.

Delving Deeper
2. Picking the Right R for the Job
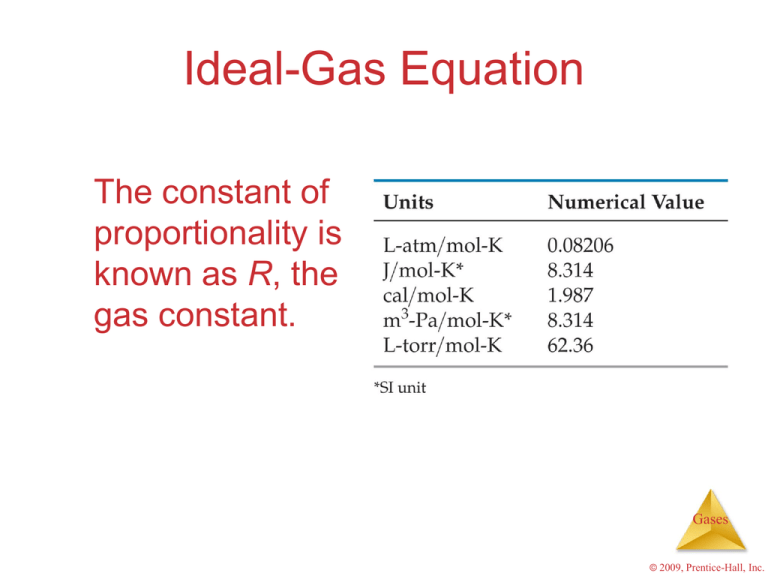
Here's the kicker: 'R' doesn't have just one value! It has different values depending on the units you're using for pressure, volume, and temperature. The most common value of 'R' that you'll likely encounter, especially when dealing with kJ, is 8.314 J/(molK). Notice the Joules? To convert that to kJ, you simply divide by 1000. This gives you R = 0.008314 kJ/(molK).
But what if your pressure is in atmospheres (atm) and your volume is in liters (L)? Then you would use a different value of 'R', namely 0.0821 Latm/(molK). Why the unit juggling? Because the ideal gas law works only when all the units are consistent. Mixing and matching units is a recipe for disaster — and incorrect answers!
So, always double-check your units before plugging in a value for 'R'. It's a small detail, but it can make a huge difference in your calculations. Think of it as making sure you have the right key for the lock. Use the wrong key (or the wrong value of 'R'), and you're not getting in anywhere!
Think of it this way: choosing the correct value for 'R' is like ordering coffee. You wouldn't order a cappuccino if you wanted an espresso, would you? Each value of 'R' is specifically tailored to a specific set of units. Understanding this is key to accurately navigating through the ideal gas law and related thermodynamic equations.

NEW KJ Ultimate Showcase (KJ Moveset Remake) YouTube
'R' in Action
3. Putting Theory into Practice
Let's say we want to calculate the amount of energy (in kJ) required to heat 2 moles of an ideal gas from 25C to 100C at constant pressure. We can use the following equation: H = n Cp T, where H is the enthalpy change (energy change), n is the number of moles, Cp is the molar heat capacity at constant pressure, and T is the change in temperature.
Cp is often related to 'R'. For an ideal gas, Cp = (5/2) R. Using R = 0.008314 kJ/(molK), we can calculate Cp = (5/2) 0.008314 kJ/(molK) = 0.020785 kJ/(molK).
Now we have all the pieces! T = 100C - 25C = 75C (or 75 K, since a change in Celsius is the same as a change in Kelvin). Plugging the values into our equation: H = 2 moles 0.020785 kJ/(molK) 75 K = 3.11775 kJ. So, it takes approximately 3.12 kJ of energy to heat the gas.
See how 'R' played a crucial role? Without it, we couldn't have determined the molar heat capacity and, therefore, couldn't have calculated the energy required. This example illustrates the practical application of 'R' in solving thermodynamic problems. This is not only helpful for science projects and calculations, but also is important to consider for real world applications such as engineering or even weather predictions!
K Buchstaben Symbol Logo Fotos Und Bildmaterial In Hoher Auflösung
Real-World Relevance
4. 'R' is Everywhere, If You Know Where to Look
You might think that 'R' is just some abstract concept confined to textbooks and laboratory experiments. But in reality, the ideal gas constant plays a vital role in many real-world applications. Consider the design of internal combustion engines. Engineers use the ideal gas law (which includes 'R') to optimize engine performance, ensuring that the fuel-air mixture burns efficiently.
Or how about climate science? Scientists use 'R' when modeling atmospheric processes, predicting weather patterns, and understanding the effects of greenhouse gases. It helps them understand how gases in the atmosphere interact and contribute to the overall climate system. It is even used to help create models and simulate the future impacts of climate change for years to come!
The food industry also relies on the ideal gas constant. For example, when packaging food under modified atmospheres (like nitrogen flushing potato chip bags), engineers use gas laws to ensure the correct gas composition for preservation. Even something as mundane as inflating a tire involves the principles governed by 'R'! So, its safe to say, 'R' is all around us.
From designing efficient engines to understanding climate change, the ideal gas constant is a cornerstone of scientific and engineering endeavors. It highlights how fundamental constants underpin many aspects of our technological world, influencing everyday conveniences and high-stakes scientific research alike.

KJ IS FREE! + Roblox Innovation Awards The Strongest
Frequently Asked Questions (FAQs)
5. Your Burning Questions Answered
Let's tackle some common questions about 'R' and its connection to kilojoules.
Q: Why does 'R' have different values?
A: Because the ideal gas law (PV = nRT) needs consistent units! Different values of 'R' accommodate different combinations of units for pressure, volume, and temperature. It's all about keeping the equation balanced.Q: Can I use 'R' if the gas isn't ideal?
A: Strictly speaking, no. 'R' is part of the ideal gas law, which assumes certain conditions (like negligible intermolecular forces). However, for many gases at moderate temperatures and pressures, the ideal gas law is a reasonable approximation. For high pressures or low temperatures, you may need to use more complex equations of state.Q: How do I convert the value of 'R' from J/(molK) to kJ/(molK)?
A: Simply divide by 1000! Since 1 kJ = 1000 J, dividing the value of 'R' in joules by 1000 gives you the equivalent value in kilojoules.Q: Is 'R' just for gases, or does it apply to liquids and solids too?
A: 'R' is primarily used in the context of gases and the ideal gas law. While there are analogous concepts and constants used in thermodynamics for liquids and solids, 'R' itself doesn't directly apply to those phases. The behaviors of liquids and solids are governed by different sets of rules due to the stronger intermolecular forces present.